Product
제품
- E.coli | 360-HCP ELISA KITS
- 제품명: E.coli | 360-HCP ELISA KITS
- 용도: Generic HCP ELISA Kit
- 메이커: Biogenes GmbH
- 카달로그:
소개
E.COLI l 360-HCP ELISA KITS Generic HCP ELISA Kit
E.coli cells are widely used for the production of biopharmaceuticals. During drug development, the analysis of residual Host Cell Proteins (HCPs) is important for ensuring the quality and safety of biopharmaceuticals. For E.coli, the HCP pattern is strongly influenced by the applied manufacturing conditions. A traditional generic assay with only one set of antibodies does not adequately represent this diversity. To overcome limitations connected to traditional generic HCP assays, BioGenes has developed the enhanced generic E.coli|360-HCP ELISA, which is composed of a set of four different generic kits.

The E.coli|360-HCP ELISA is an enhanced generic host cell protein (HCP) assay. It was designed to cover a broader spectrum of E.coli host cell proteins (HCPs) than other commercially available generic HCP assays.
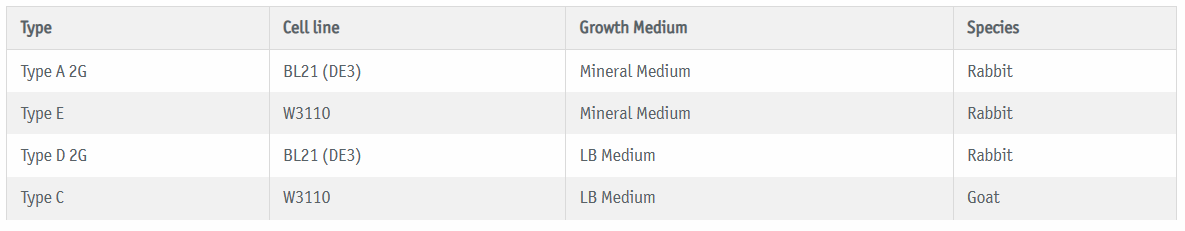
E.coli|360-HCP ELISA is based on W3110 and BL21 (DE3) cell lines which were fermented in different culture media and conditions resulting in several antigen preparations with distinct HCP patterns. By immunizing goats (type C) or rabbits (types A, D and E) with these antigens, BioGenes has developed a panel of different HCP ELISAs kits that together build up the enhanced generic E.coli|360-HCP ELISA.
High specificity of the antibody preparations was demonstrate by coverage of ≥ 80% of the respective E.coli|360-HCP antigen distribution. Antigen coverage was measured by a 2D Western blot with Cy5-labeled E.coli|360-HCP standard and the corresponding Cy3-labeled anti-HCP antibodies.
Satisfying recovery was estimated in spiked samples within the assay working range within the acceptance criterion 100±30% of spiked antigen. High sensitivity of E.coli|360-HCP ELISA kits, described by the validation parameter LOD, LOQ and working range, is shown below:
• LOD : 0.2 – 0.5 ng/mL
• LOQ : 0.6 – 1.6 ng/mL
• Working range : 2 – 100 ng/mL
The HCP pattern in E.coli is influenced by many factors
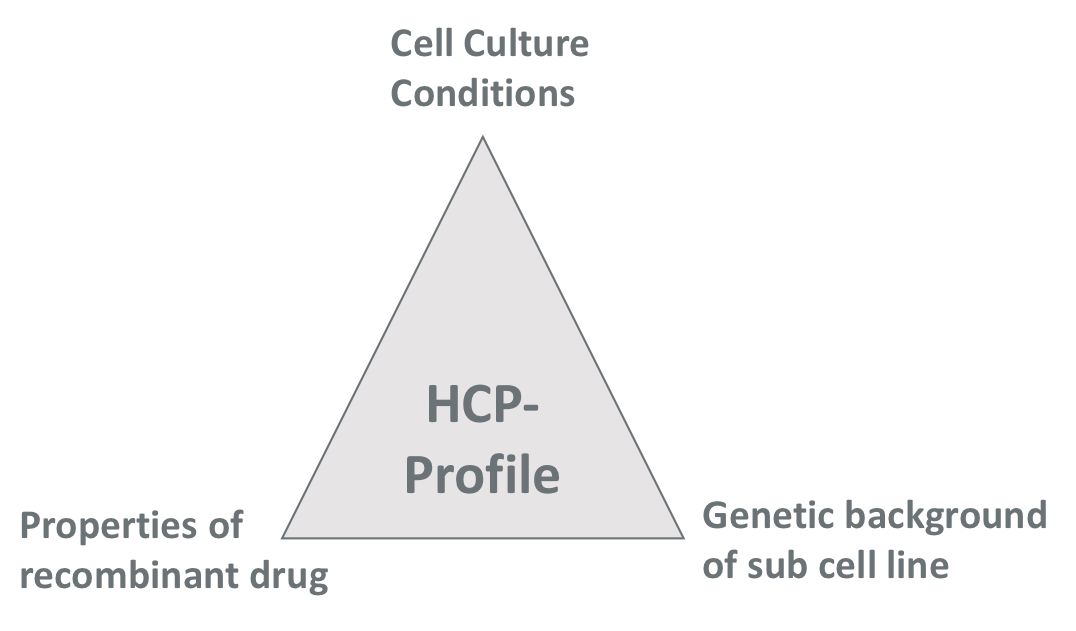
1) Genetic background of E.coli strains
The genomes of different E.coli strains show remarkable diversity. The typical E.coli genome encodes for approx. 4,500 - 5,000 genes, from which approx. 50 % of the genes are represented in the core genome. It is estimated that the pan-genome of E.coli comprises approx. 128,000 genes, and thus carries a large number of potential HCP species.
2) Cell cultivation conditions
The gene expression of E.coli is highly dependent on the growth phase of the cells, type of media and supplements and growth conditions. The re-organization of central metabolic pathways, the expression of stress-related genes and the secretion of enzymes for substrate-degradation are mainly influenced by the availability of nutrients and the presence of cell stress conditions such as temperature, oxygen and salinity.
3) Properties of the recombinant drug
The over-expression of a recombinant (foreign) protein can lead to significant changes in the proteome of the recombinant strain with respect to the expression patterns of metabolically functional proteins, chaperones and heat shock proteins.
Why four kit types?
All these conditions together influence the unique HCP profile which occurs during E.coli fermentation, and no single generic kit will be able to detect every HCP.
The E.coli|360-HCP ELISA provides increased assay specificity and sensitivity with different sets of anti-HCP antibodies, organized into four kit types (Type A, Type C, Type D, Type E), for optimized HCP detection.
• Genetic background of the E.coli antigens: BL21 (DE3) & W3110
• Fermentation media: LB Medium & Mineral Medium
Due to the difficulties of predicting the HCP spectrum in E.coli, we recommend testing all four kit types first and then selecting the best performing kit according to ELISA key parameters, such as dilution linearity, accuracy and total coverage.
How does the generic ELISA kit generation work
The manufacturing of each enhanced generic HCP ELISA kit type involves the following steps:
• Immunization of individuals using particular antigen material
• Isolation of polyclonal antibodies and purification
• Manufacturing of ready-to-use ELISA kits
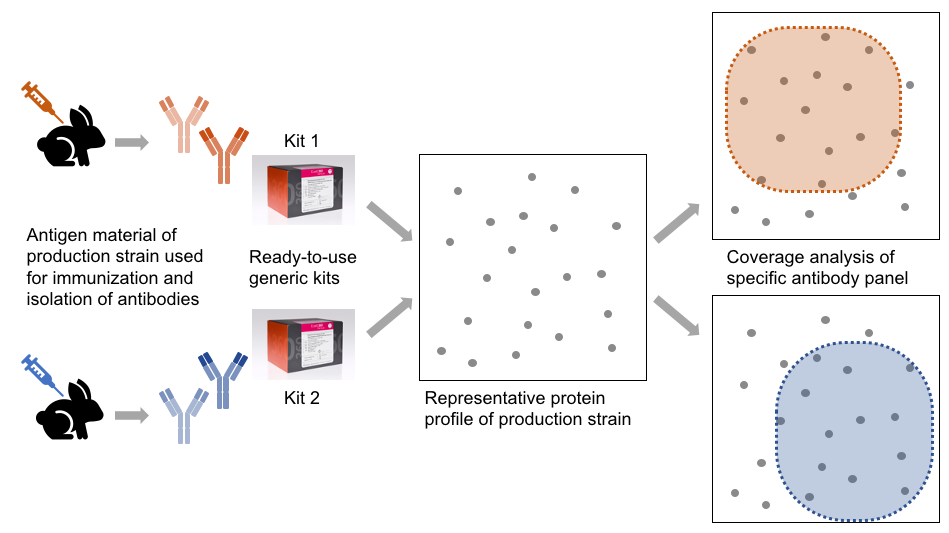
The antibodies of each individual kit type will display specific reactivity towards a subset of the total protein profile and will show weak or no reactivity towards other proteins of given strain/cell line. The proportion of proteins being recognized compared to the total protein is termed coverage.
How to select the kit with the best fit?
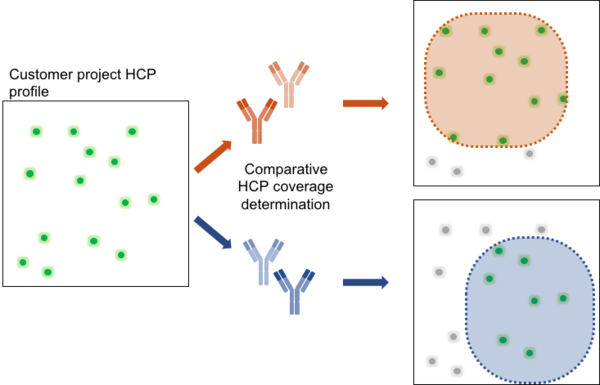
Each drug manufacturing process results in a particular HCP profile. To achieve optimal HCP monitoring, a testing for best fit should be applied. As depicted, the antibody panel of the upper kit covers a broader range of HCPs in the exemplary project sample compared to the kit on the bottom. Thus, the upper kit would be the better choice to achieve monitoring of HCP reduction due to better overall coverage. For the identification of the generic 360 HCP ELISA kit with the best fit, BioGenes recommends to apply testing for
Critical HCP ELISA performance criteria
• HCP coverage
• Sufficient HCP log-reduction
• ELISA accuracy
• Sample dilution linearity
동영상
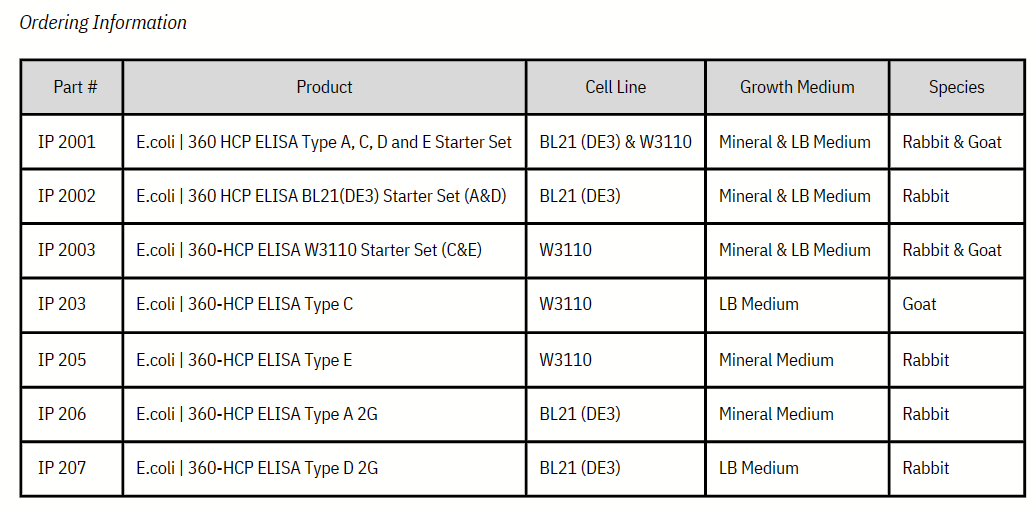
주문정보
Each kit type contains :
• Pre-coated 96-well microtest plate
• Standard E.coli-HCP
• Antibody conjugate
• Enzyme conjugate
• Washing buffer
• Assay buffer
• Substrate buffer
• Stop solution
관련자료


