Product
제품
- CHO | 360-HCP ELISA KITS
- 제품명: CHO | 360-HCP ELISA KITS
- 용도: Generic HCP ELISA Kit
- 메이커: Biogenes GmbH
- 카달로그:
소개
CHO l 360-HCP ELISA KITS Generic HCP ELISA Kit
The Chinese hamster ovary (CHO) cell line is the most prevalent biopharmaceutical expression system, and has been proven safe for the commercial production of protein therapeutics. To ensure the safety of recombinant-expressed biopharmaceuticals, health authorities are demanding the close monitoring of HCP impurities by applying HCP detection methods with high sensitivity and specificity. The CHO|360-HCP ELISA is an enhanced generic HCP assay composed of four different generic kits, which was designed to cover a broader spectrum of CHO-HCPs compared to single generic kits.


The CHO|360-HCP ELISA kits (Type A to D) are based on a unique assay design, employing differently-prepared HCP antigens and antibodies from two species:
• The antigen is prepared from total and fractionated HCP obtained from mock fermentation of CHO-K1 and CHO-S
• The specific antibodies are generated by parallel immunization of goats and rabbits
This approach results in four different HCP ELISA kits, which together make the enhanced generic CHO|360-HCP assay.
The CHO|360-HCP ELISA method was qualified at BioGenes according to the respective ICH-Guideline ICH Q2(R1) (“Validation of analytical procedures: text and methodology“) with slightly deviations due to the nature of the method as a bioassay.
High specificity of all four antibody preparations (A to D) was demonstrated by a coverage of ≥ 80% of the CHO|360-HCP antigen distribution in 1D and 2D analysis. For each CHO|360-HCP ELISA kit (A to D), satisfying recovery was estimated in the three spiked samples within the assay working range, within the acceptance criterion 100±30% of spiked antigen. High sensitivity of CHO|360-HCP ELISA Kits (A to D), described by the validation parameter LOD, LOQ and working range, is shown below:
• LOD : 0.5 – 1.0 ng/mL
• LOQ : 2 – 3 ng/mL
• Working range: 2 – 100 ng/mL
HCP recovery strongly depends on the type of ELISA kit used
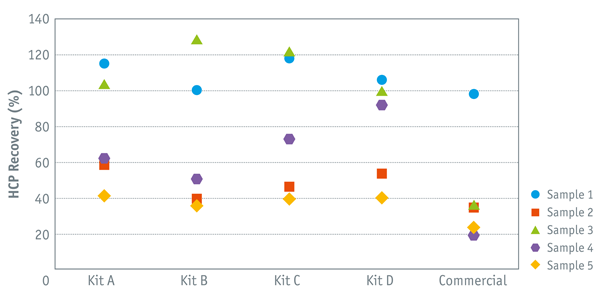
To show this, we tested CHO|360-HCP ELISA kits (A to D) and a commercially available generic CHO HCP assay, on the basis of a great number of mock CHO HCP samples. In most cases, the best recovery was achieved with one of the four CHO|360-HCP ELISAs kits, as compared to other commercial kits (see right-hand image).
• Sample 4: a recovery value of > 90% was estimated with ELISA kit type D while ELISA kits A, B and C and the commercial kit
were less sensitive, with the latter detecting only 20% of the host cell proteins.
• Recovery values > 100% may occur due to overestimation.
Why does BioGenes apply HCP fractionation prior to immunization?
Naturally, low molecular weight (LMW) proteins are less immunogenic than larger proteins, leading to a reduced production of anti-LMW HCP antibodies after the immunization of animals. Consequently, very weakly immunogenic or non-immunogenic HCPs may not be detected by ELISA or Western Blot. The BioGenes'CHO|360-HCP ELISA solved this by using the total and fractionated HCP for the immunization of animals, resulting in four different HCP ELISA kits (A to D).
Antibodies obtained from immunization with each of three different molecular-weight fractions were then pooled again to combine antibody reactivity towards to the complete HCP spectrum. The immunization of rabbits and goats enables the generation of distinct antibody panels, which allows for a broader coverage of HCPs in customized samples.
The BioGenes recommendation:
We recommend testing all four kit types (Starter Set) first, and then selecting the best performing kit according to ELISA key parameters (such as dilution linearity, accuracy and total coverage) for use in all subsequent tests.
How does the generic ELISA kit generation work
The manufacturing of each enhanced generic HCP ELISA kit type involves the following steps:
• Immunization of individuals using particular antigen material
• Isolation of polyclonal antibodies and purification
• Manufacturing of ready-to-use ELISA kits
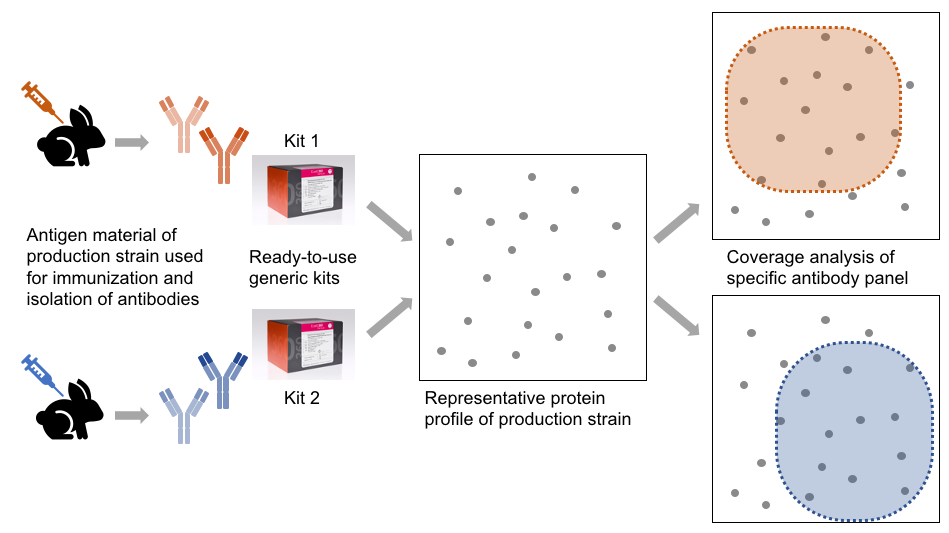
The antibodies of each individual kit type will display specific reactivity towards a subset of the total protein profile and will show weak or no reactivity towards other proteins of given strain/cell line. The proportion of proteins being recognized compared to the total protein is termed coverage.
How to select the kit with the best fit?
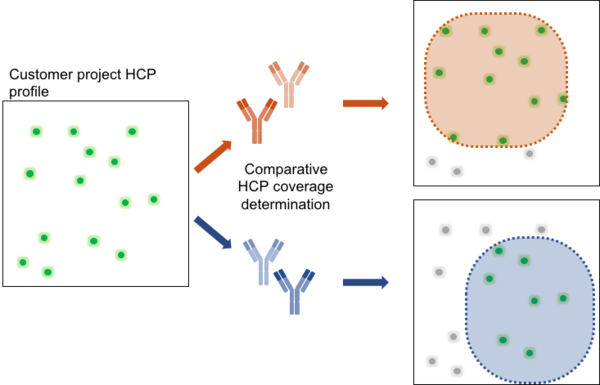
Each drug manufacturing process results in a particular HCP profile. To achieve optimal HCP monitoring, a testing for best fit should be applied. As depicted, the antibody panel of the upper kit covers a broader range of HCPs in the exemplary project sample compared to the kit on the bottom. Thus, the upper kit would be the better choice to achieve monitoring of HCP reduction due to better overall coverage. For the identification of the generic 360 HCP ELISA kit with the best fit, BioGenes recommends to apply testing for
Critical HCP ELISA performance criteria
• HCP coverage
• Sufficient HCP log-reduction
• ELISA accuracy
• Sample dilution linearity
동영상
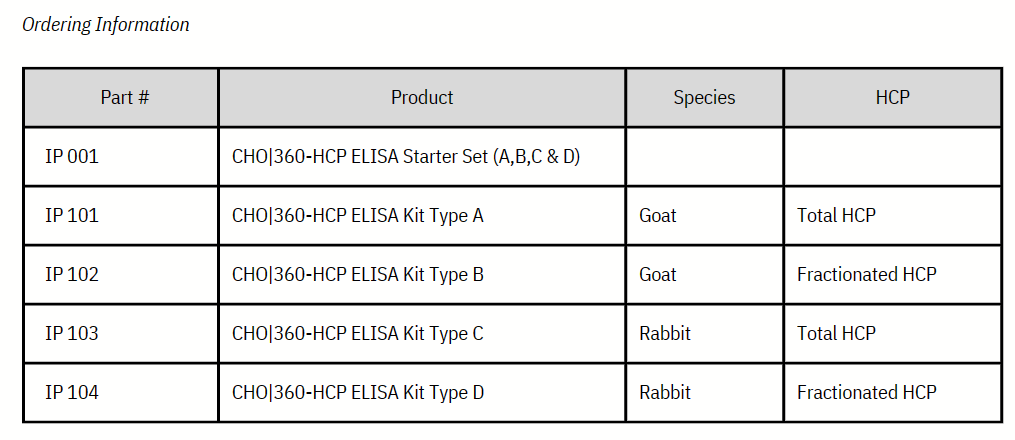
주문정보
Each kit type contains :
• Pre-coated 96-well microtest plate
• Standard E.coli-HCP
• Antibody conjugate
• Enzyme conjugate
• Washing buffer
• Assay buffer
• Substrate buffer
• Stop solution
관련자료


