Product
제품
-
Analytical Products
- Wyatt Technology
- Awareness Technology
- Eurofins l Abraxis (GSD)
- Aurora Biomed
- Canada NRC-CNRC
- Chrom Tech
- Eichrom Technologies
- EPROGEN
- Fluidic Analytics
- Globe Scientific
- GENERON
- Halo Labs
- Hygiena International
- KROMATON
- InProcess-LSP
- MTC Bio
- MZ-Analysentechnik
- Newomics Inc.
- Occhio Instruments
- Optimize Technologies
- Pickering Laboratories
- PolyLC
- Raykol Group
- RheoSense
- Rocker Scientific
- Santai Science
- SEDERE
- Spectra Analysis
- UCT
- Wealtec Corp
-
Bio & Medical Products
- Biolog
- Adooq Bioscience
- A&A Biotechnology
- Accegen Biotechnology
- Anatrace
- Array Bridge
- Biogenes GmbH
- BioQuochem
- BioServ UK
- Biomiga
- Biotech Support Group
- CinderBio
- Cell Technology
- Creative Biolabs
- Creative Diagnostics
- Creative Biostructure
- Creative Biomart
- Creative Enzymes
- EICOM
- Emulseo
- GLYcoDiag
- Helix Biotech
- InnoGenomics
- IsoSciences, LLC
- IUL Instruments
- Micropore Technologies
- Matrix Innovation
- PreciGenome
- PhylumTech
- ProFoldin
- Protein Ark
- Primer Design
- ProteoChem
- RareCyte
- RECIPE
- Silicycle Inc.
- Tymora Analytical
- UTAK
- YouSeq
- Z Biotech
- Apo3HTS
- 제품명: Apo3HTS
- 용도: High Throughput Screen for Caspase 3/7 Detection
- 메이커: Cell Technology
- 카달로그:
소개
Apo3HTS
High Throughput Screen for Caspase 3/7 Detection
1. Key Benefits
• Homogenous assay for active caspase 3/7
• Breakthrough in cell lysis buffer and preservation of caspase activity
• Results in a no-wash, one-step assay
• No need to wash out media from cell samples, just add the reagent directly to your experimental samples
• Easy to Use: No need to make cell lysates or run Western blots
• Works with suspension and adherent cells
2. Assay Principle
Cell Technology’s APO 3/7 HTS Assay utilizes the quenched (z-DEVD)2-R110 peptide substrate for caspase 3/7 detection. The absorption and emission properties of the R110 dye are suppressed when attached to the z-DEVD peptide sequence. When R110 is cleaved away, by active caspase3/7, form the quenching DEVD sequence, the free dye excites at 488nm and emits at 515-530 nm.
As a result of a novel and proprietary Lysis Buffer System, the APO 3/7 HTS Assay is a homogenous platform that can be utilized for high throughput fluorescence plate reader applications. The reagent is directly added to the samples thus eliminating any wash steps.
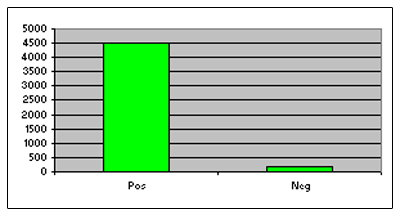
Figure. In this figure, Jurkat cells were stimulated with various concentrations of staurosporine for 3 hours, after which caspase 3/7 activity was analyzed using the APO 3/7 HTS kit.
주문정보
|
Reagent |
Catalog # |
Size |
|
Apo 3/7 HTS |
APO200-2 |
100 tests |
|
Apo 3/7 HTS |
APO200-3 |
500 tests |
|
Apo 3/7 HTS |
APO200-4 |
1000 tests |
관련자료
Reference
Slee, E. A., C. Adrain, and S. J. Martin. 1999. Serial Killers: ordering caspase activation events in apoptosis. Cell Death and Differ. 6:1067-1074.
Walker, N. P., R. V. Talanian, K. D. Brady, L. C. Dang, N. J. Bump, C. R. Ferenz, S. Franklin, T. Ghayur, M. C. Hackett and L. D.Hammill. 1994. Crystal Structure of the Cysteine Protease Interleukin-1ß-Converting Enzyme: A (p20/p10)2 Homodimer. Cell 78:343-352.
Wilson, K. P., J. F. Black, J. A. Thomson, E. E. Kim, J. P. Griffith, M. A.Navia, M. A. Murcko, S. P. Chambers, R. A. Aldape, S. A. Raybuck, and D. J.Livingston. 1994. Structure and mechanism of interleukin-1 beta converting enzyme. Nature 370: 270-275.
Rotonda, J., D. W. Nicholson, K. M. Fazil, M. Gallant, Y. Gareau, M. Labelle,E. P. Peterson, D. M. Rasper, R. Ruel, J. P. Vaillancourt, N.A. Thornberry and J.W. Becker. 1996. The three-dimensional structure of apopain/CPP32, a key mediator of apoptosis. Nature Struct. Biol. 3(7): 619-625.
Kumar, S. 1999. Mechanisms mediating caspase activation in cell death. Cell Death and Differ. 6: 1060-1066.
Alnemri, E.S. et al (1996) Cell 87:171
Trends Biochem Sci 22,388 (1997)
