Product
제품
- PMF4
- 제품명: PMF4
- 용도: Fluorescent Formaldehyde Detection Kit (Cell Permeable)
- 메이커: Cell Technology
- 카달로그:
소개
PMF4
Fluorescent Formaldehyde Detection Kit (Cell Permeable)
Traditionally, formaldehyde (FA) has been viewed as an environmental hazard and a toxic carcinogen for mammals. However, FA is produced in mammalian cells via enzymatic pathways and used in the “one carbon cycle” to make building blocks for life such as DNA and certain amino acids. This ‘one carbon cycle’ is fundamental to all forms of life down to the bacterial cell (1-6). FA is maintained in homeostasis in living cells, however its breakdown and over production has been implicated in the pathogenesisof various diseases such as cancer, dementia, diabetes and Alzheimer’s disease (7-11).
Cell Technology introduces two ultra-fast reversible probes, developed by Dr. Xin Li et al, PMF and PMF4 for the detection of FA in living cells or aqueous samples (12,13). These quenched fluorogenic probes when exposed to FA become fluorescent: Ex 320-420nm Em: 520-560mm. PMF4 is especially useful in in-situ visualization of FA in cellular lysosomes.
1. Key Benefits
• Probe PMF: In-situ monitoring of Formaldehyde (FA) in the cytoplam
• Probe PMF4: In-situ monitoring of Formaldehyde (FA) in lysosomes
• Study of FA levels in cellular stress, diseases such as Alzhimers and cancer
• Reversible probe monitoring FA homeostasis in live cells and tissue sections
• FA detection in Bacterial, fungal and plant cells
• Environmental samples.
2. Assay Principle
PMF and PMF4 are quenched fluorogenic probes and when exposed to FA become fluorescent: These probes are extremely useful for continuous in-situ monitoring of FA in live cells or live tissue sections. Ex 320-420nm Em: 520-560mm. PMF4 is especially useful in in-situ visualization of FA in cellular lysosomes.
Reaction:
1. PMF/PMF4 Non-Fluorescent Dye + Formaldehyde → Fluorescent Analog
Excitation: 320-420nm and Emission at 520-560nm. Flow Cytometery /Fluorescent Microscope Ex: 488 Em: 520-560nm.
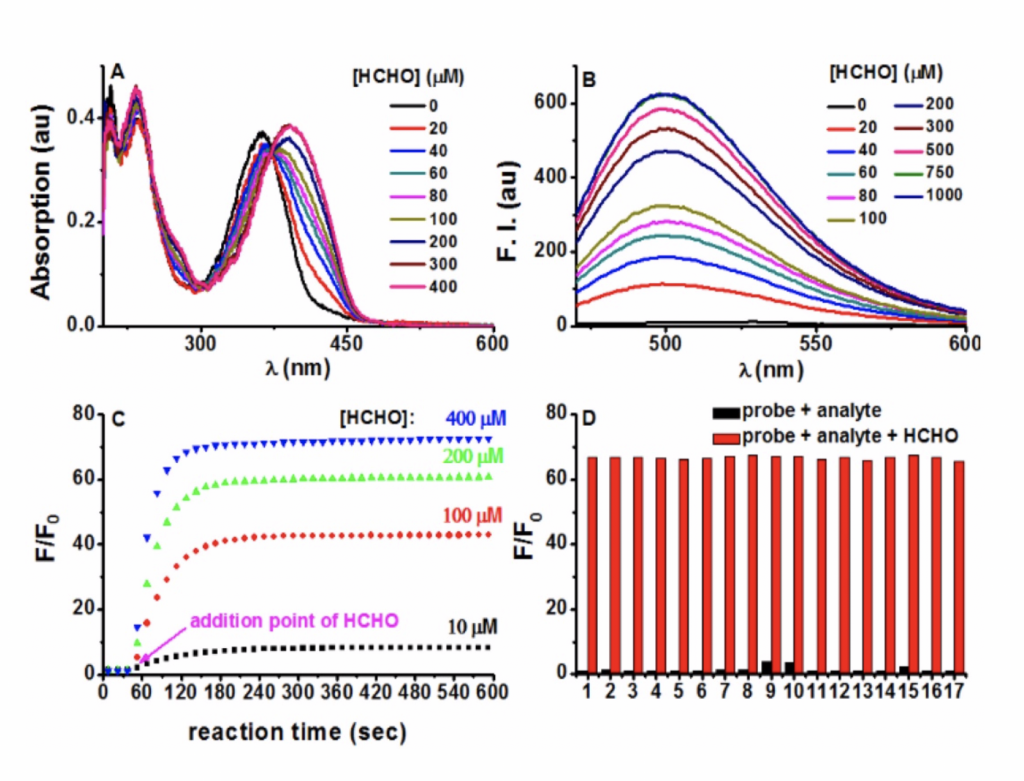
Figure. Photophysical responses of PFM to FA. (A) PFM (20 μM) was treated with various amounts of FA for 5 min, and then the UV−vis spectra were recorded. (B) Fluorescent spectra of PFM (10 μM) after the treatment of various concentrations of FA for 5 min. (C) Reaction time course of PFM (10 μM) with FA (10, 100, 200, 400 μM). (D) Fluorescent responses of PFM (10 μM) toward various analytes (300 μM) after a reaction time of 30 min without (black) or with (red) the presence of FA (300 μM). The analytes tested were PFM blank (1), acetaldehyde (2), malonaldehyde (3), ascorbic acid (4), glucose (5), glucosone (6), oxalic acid (7), pyruvate (8), methylglyoxal (9), glyoxal (10), p-methoxybenzaldehyde (11), trichloroacetaldehyde (12), p-nitrobenzaldehyde (13), acetone (14), HClO (15), H2O2 (16), GSH (17). For C and D, F represents the fluorescent intensity of PFM at 500 nm after the treatment of FA for various time or after the treatment of various analytes, and F0 represents the intensity of blank PFM solution at 500 nm. All data were collected in PBS (pH 7.4, 10 mM) at ambient temperature with λex 451 nm.
3. Applications
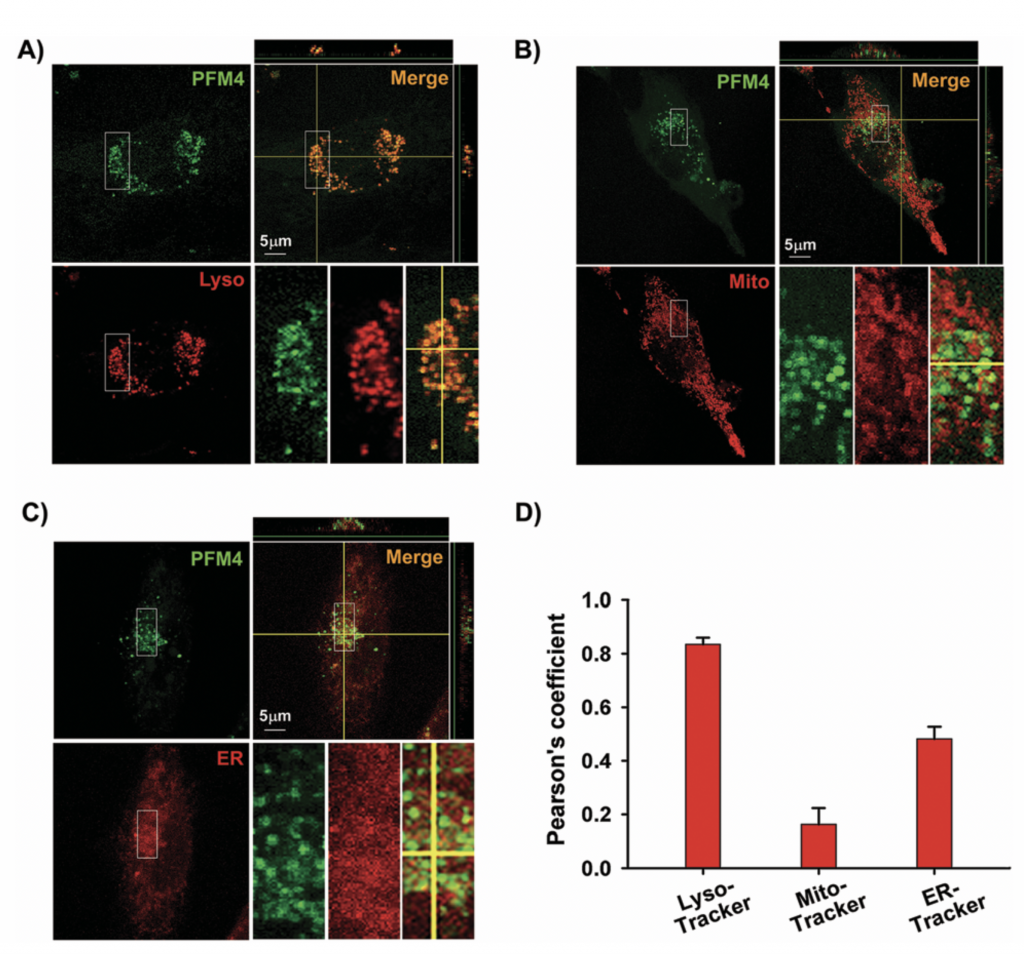
Figure. Confocal microscopic images of subcellular localization of PFM4 in EA cells. (A) Cells were incubated with PFM4 (5 μM, green) for 15 min at 370C and subsequently stained with 1 mM Lyso-Tracker (red, A), or Mito- Tracker (red, B), or ER-Tracker (red, C). Orthogonal projection onto the x–z and y–z panels as shown to confirm the co-localization of PFM4 with the organelle trackers. Cells in the bottom-right picture are the magnification of the panel in the top row. The PFM4 fluorescence was monitored at lem = 475–560 nm (lex = 458 nm). Fluorescent signals from Lyso-Tracker/ Mito-Tracker/ER-Tracker (red) were obtained at lem = 587–680 nm (lex = 543 nm). Scale bars = 5 mm. (D) Co-localization of PFM4 with lysosomes, mitochondria and endoplasmic reticulum were assessed using the Pearson’s coefficient.
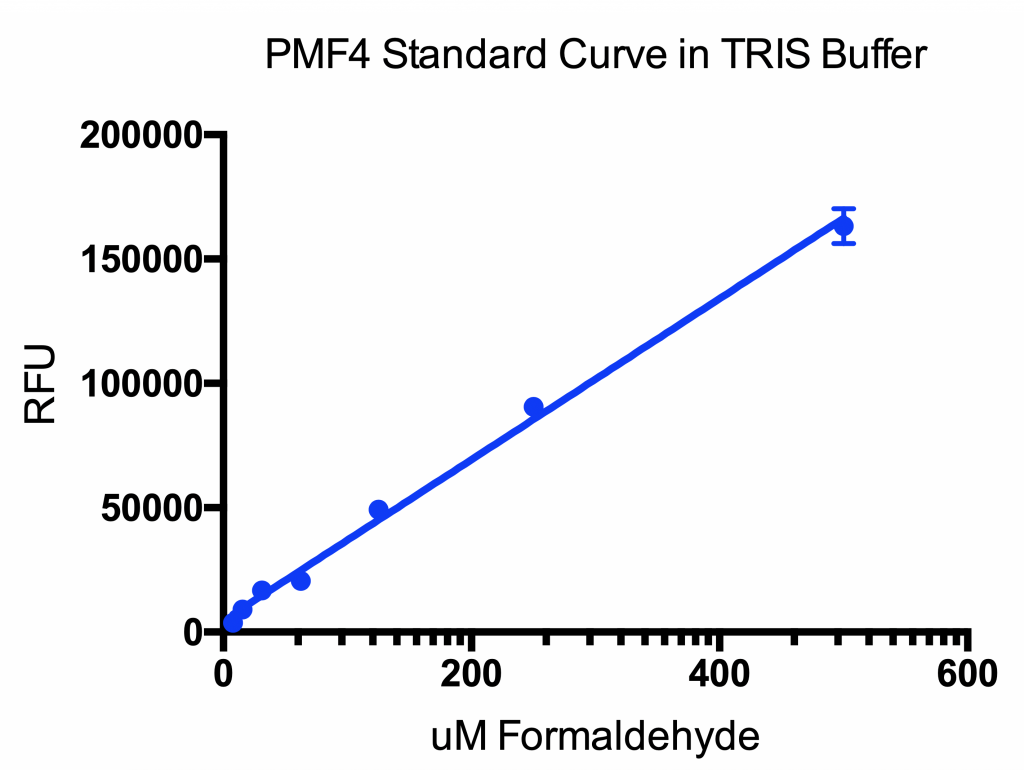
Figure. Sample of Standard curve of formaldehyde titrated in 100mM TRIS buffer pH 7.0. PMF4 added at a concentration of 10μM final concentration per well. The assay plate was incubated for 15 minutes in the dark at room temperature. Ex:360nm and Em: 520nm.
주문정보
|
Reagent |
Catalog # |
Size |
|
PMF4: 1 vial |
PMF4-100-2 |
0.5mg |
|
PMF4: 1 vial |
PMF4-100-3 |
2.0mg |
관련자료
Reference
Yu PH, Wright S, Fan EH, Lun ZR, Gubisne-Harberle D. Physiological and pathological implications of semicarbazide-sensitive amine oxidase. Biochim Biophys Acta. 2003; 1647: 193-9.
O'Sullivan J, Unzeta M, Healy J, O'Sullivan MI, Davey G, Tipton KF. Semicarbazide-sensitive amine oxidases: enzymes with quite a lot to do. Neurotoxicology. 2004; 25: 303-15
Lee ES, Chen H, Hardman C, Simm A, Charlton C. Excessive S-adenosyl-L-methionine-dependent methylation increases levels of methanol, formaldehyde and formic acid in rat brain striatal homogenates: possible role in S-adenosyl-L-methionine-induced Parkinson's disease-like disorders. Life Sci. 2008; 83: 821-7.
Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, Casero RA, Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 2004; 119: 941-53.
WangY, ZhangH, ChenY, SunY, YangF, YuW,LiangJ, SunL, YangX, ShiL, Li R, Li Y, Zhang Y, Li Q, Yi X, Shang Y. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell 2009; 138: 660-72. 6. Guillermo Burgos-Barragan, Niek Wit, Johannes Meiser, Felix A. Dingler, Matthias Pietzke, Lee Mulderrig, Lucas B. Pontel, Ivan V. Rosado, Thomas F. Brewer, Rebecca L. Cordell, Paul S. Monks, Christopher J. Chang, Alexei Vazquez, Ketan J. Patel. Mammals divert endogenous genotoxic formaldehyde into one-carbon metabolism. Nature, 2017;
A. R. Hipkiss, Aging Dis., 2017, 8, 128–130.
R. Baan, Y. Grosse, K. Straif, B. Secretan, F. El Ghissassi, V. Bouvard, L. Benbrahim-Tallaa, N. Guha, C. Freeman, L. Galichet, V. Cogliano and W. H. O. I. A. f. R. o. C. M. W. Group, Lancet Oncol., 2009, 10, 1143–1144.
J. D. Rizak, Y. Ma and X. Hu, Curr. Alzheimer Res., 2014, 11, 461–468. 20 J. Lu, J. Miao, T. Su, Y. Liu and R. He, Biochim. Biophys. Acta, 2013, 1830, 4102–4116.
T. Jiang, Q. Sun and S. Chen, Prog. Neurobiol., 2016, 147, 1–19.
A. Rahmadi, N. Steiner and G. Munch, Clin. Chem. Lab. Med., 2011.
Xing-Guang Liang, Bo Chen, Ling-Xiao Shao, Juan Cheng, Ming-Zhu Huang, Yu Chen, Yong-Zhou Hu, Yi-Feng Han, Feng Han and Xin Li. A Fluorogenic Probe for Ultrafast and Reversible Detection of Formaldehyde in Neurovascular Tissues. Theranostics, 2017:7(8): 2305-2313. doi: 10.7150/thno.19554
